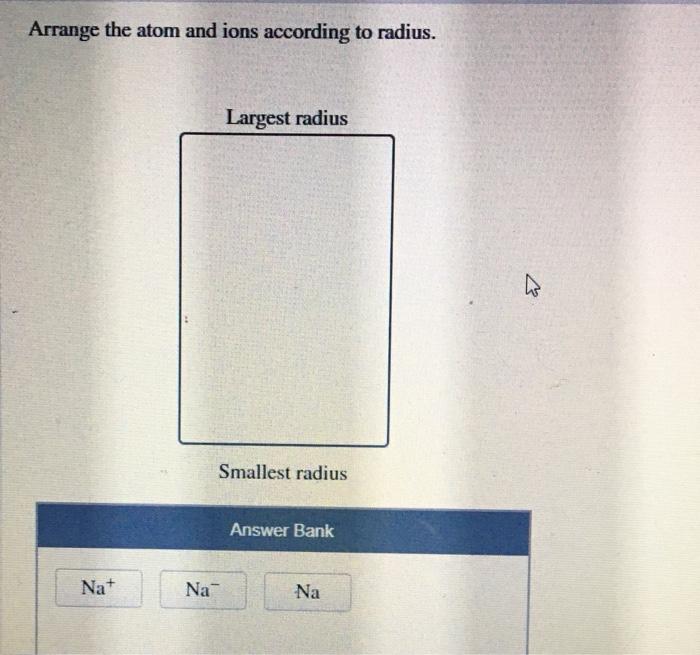
Arrange the atom and ions according to radius. – Atomic and ionic radii are fundamental properties that govern the behavior of atoms and ions, influencing their chemical and physical characteristics. Understanding these radii is crucial in various scientific disciplines, including chemistry, materials science, and biology. This comprehensive guide delves into the concept of atomic and ionic radii, exploring their trends, factors affecting them, and their wide-ranging applications.
Atomic radius refers to the distance from the nucleus to the outermost electron shell of an atom, while ionic radius pertains to the size of an ion, which differs from its atomic counterpart due to the gain or loss of electrons.
Factors such as the number of electrons, effective nuclear charge, and ionic charge significantly impact these radii.
Atomic Radius
Atomic radius is the distance from the nucleus to the outermost electron shell of an atom. It is measured in picometers (pm), which are one trillionth of a meter. The atomic radius of an element can be determined by a variety of experimental techniques, such as X-ray crystallography and electron diffraction.
The atomic radius of an element is affected by a number of factors, including the number of electrons in the atom and the effective nuclear charge. The effective nuclear charge is the net positive charge experienced by the electrons in the atom.
It is equal to the number of protons in the nucleus minus the number of electrons in the inner electron shells.
Atoms with more electrons have larger atomic radii than atoms with fewer electrons. This is because the electrons in the outer electron shells are less strongly attracted to the nucleus than the electrons in the inner electron shells. The effective nuclear charge also affects the atomic radius.
Atoms with a higher effective nuclear charge have smaller atomic radii than atoms with a lower effective nuclear charge. This is because the higher effective nuclear charge attracts the electrons in the outer electron shells more strongly.
Examples of Atoms with Different Atomic Radii
- Hydrogen: 53 pm
- Carbon: 70 pm
- Nitrogen: 75 pm
- Oxygen: 80 pm
- Fluorine: 95 pm
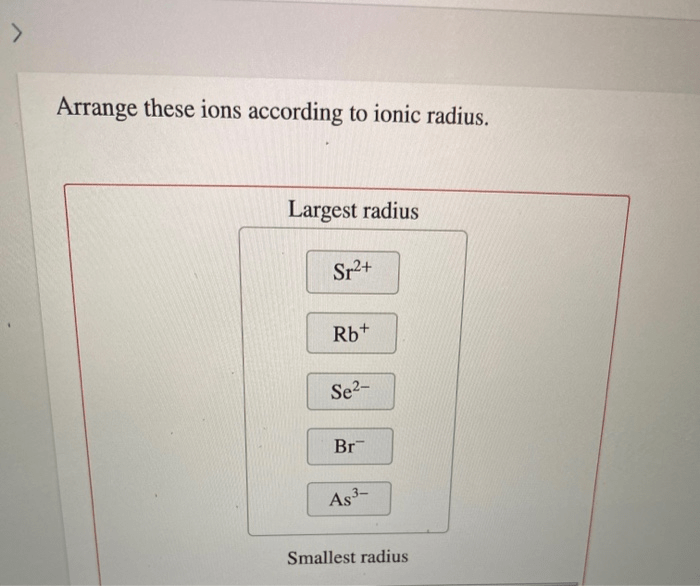
Ionic Radius
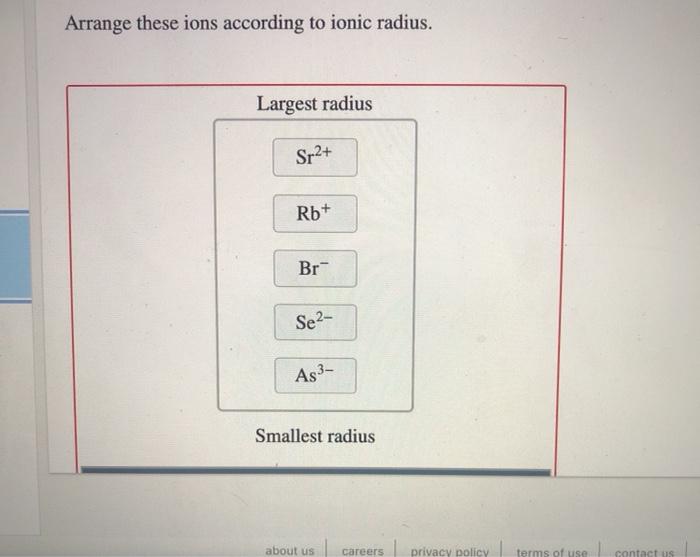
Ionic radius is the distance from the nucleus to the outermost electron shell of an ion. It is measured in picometers (pm), which are one trillionth of a meter. The ionic radius of an ion is different from the atomic radius of the corresponding atom.
This is because when an atom becomes an ion, it either gains or loses electrons. This changes the number of electrons in the atom, which in turn changes the effective nuclear charge. The effective nuclear charge of an ion is equal to the number of protons in the nucleus minus the number of electrons in the ion.
Ions with more electrons have larger ionic radii than ions with fewer electrons. This is because the electrons in the outer electron shells are less strongly attracted to the nucleus than the electrons in the inner electron shells. The effective nuclear charge also affects the ionic radius.
Ions with a higher effective nuclear charge have smaller ionic radii than ions with a lower effective nuclear charge. This is because the higher effective nuclear charge attracts the electrons in the outer electron shells more strongly.
Examples of Ions with Different Ionic Radii
- Na +: 95 pm
- Cl –: 181 pm
- O 2-: 140 pm
- Ca 2+: 99 pm
- F –: 133 pm
Trends in Atomic and Ionic Radii
The atomic and ionic radii of elements vary across the periodic table. In general, the atomic and ionic radii of elements increase from right to left across a period and decrease from top to bottom within a group. This is because the number of electrons in an atom increases from right to left across a period and the number of electron shells increases from top to bottom within a group.
The atomic and ionic radii of elements can be used to predict the properties of compounds. For example, compounds formed between elements with large atomic and ionic radii tend to be more ionic than compounds formed between elements with small atomic and ionic radii.
This is because the larger the atomic and ionic radii, the weaker the electrostatic attraction between the ions.
Table of Atomic and Ionic Radii, Arrange the atom and ions according to radius.
| Element | Atomic Radius (pm) | Ionic Radius (pm) |
|---|---|---|
| Hydrogen | 53 | – |
| Carbon | 70 | – |
| Nitrogen | 75 | – |
| Oxygen | 80 | – |
| Fluorine | 95 | – |
| Sodium | 155 | 95 |
| Chlorine | 99 | 181 |
| Potassium | 235 | 133 |
| Calcium | 197 | 99 |
Applications of Atomic and Ionic Radii

The atomic and ionic radii of elements are used in a variety of fields, including chemistry, materials science, and biology.
In chemistry, the atomic and ionic radii of elements are used to predict the properties of compounds. For example, compounds formed between elements with large atomic and ionic radii tend to be more ionic than compounds formed between elements with small atomic and ionic radii.
This is because the larger the atomic and ionic radii, the weaker the electrostatic attraction between the ions.
In materials science, the atomic and ionic radii of elements are used to design new materials. For example, materials with a high thermal conductivity are often made from elements with large atomic and ionic radii. This is because the larger the atomic and ionic radii, the more easily heat can flow through the material.
In biology, the atomic and ionic radii of elements are used to understand the structure and function of biological molecules. For example, the atomic and ionic radii of elements are used to predict the shape of proteins. This is because the shape of a protein is determined by the way in which its amino acids are folded.
The amino acids in a protein are folded in such a way that the atomic and ionic radii of the elements in the amino acids are maximized.
Essential FAQs: Arrange The Atom And Ions According To Radius.
What is the relationship between atomic radius and ionic radius?
Ionic radius is generally smaller or larger than atomic radius, depending on whether the ion is a cation (positively charged) or an anion (negatively charged).
How do atomic and ionic radii affect chemical bonding?
Atomic and ionic radii influence the strength and type of chemical bonds formed between atoms and ions. Smaller radii promote stronger bonds, while larger radii weaken them.
What are the applications of atomic and ionic radii in materials science?
Understanding atomic and ionic radii is essential in designing materials with specific properties, such as semiconductors, ceramics, and alloys.