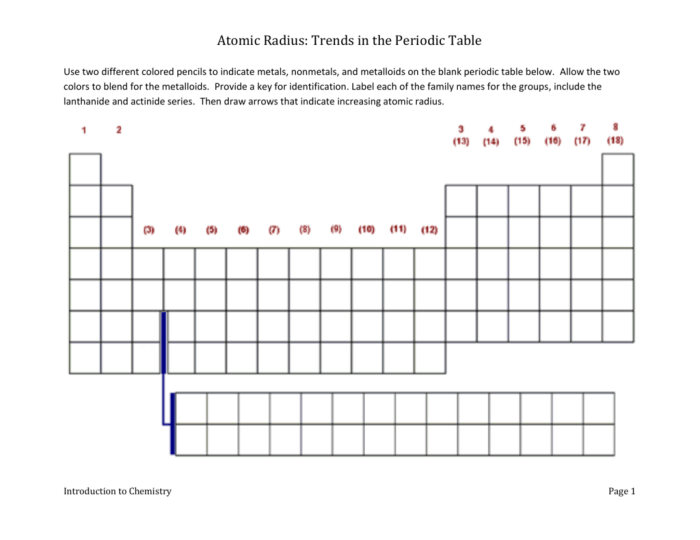
Periodic trends worksheet atomic radius – Embark on an exploration of atomic radius trends with our comprehensive worksheet, “Periodic Trends Worksheet: Delving into Atomic Radius.” This educational resource provides a thorough understanding of atomic radius, its significance, and its fascinating periodic variations.
As we traverse the periodic table, we will uncover the factors that shape atomic radius, including atomic number, electron configuration, and shielding effects. Through engaging examples and interactive exercises, you will gain a deep appreciation for the interplay between these factors and their impact on atomic size.
Periodic Trends in Atomic Radius: Periodic Trends Worksheet Atomic Radius
Introduction
Atomic radius is a fundamental property of an element that represents the average distance from the nucleus to the outermost electron shell. It plays a crucial role in determining the chemical and physical properties of elements and their interactions with other atoms.
Periodic trends are patterns observed in the properties of elements when arranged in the periodic table. Understanding these trends provides valuable insights into the behavior and reactivity of elements.
Factors Affecting Atomic Radius
Several factors influence atomic radius, including:
- Atomic number:As atomic number increases, the number of protons in the nucleus increases, resulting in a stronger attraction between the nucleus and the electrons. This leads to a decrease in atomic radius.
- Electron configuration:The number and arrangement of electrons in the outermost energy level affect atomic radius. Elements with more electrons in the outermost shell have larger atomic radii due to increased electron-electron repulsion.
- Shielding effect:The shielding effect refers to the ability of inner electrons to reduce the attraction between the nucleus and the outermost electrons. The presence of more inner electrons shields the outermost electrons from the nuclear charge, resulting in a larger atomic radius.
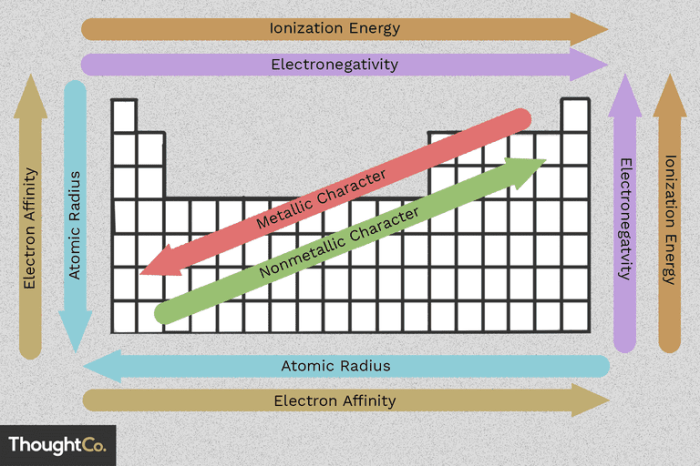
Periodic Trends in Atomic Radius
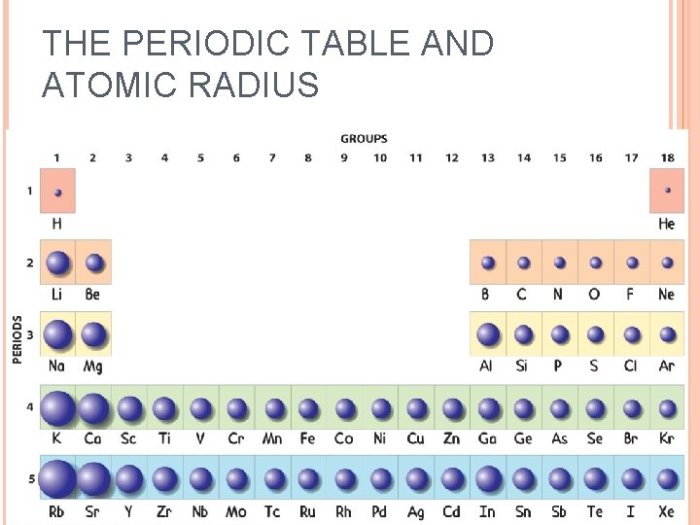
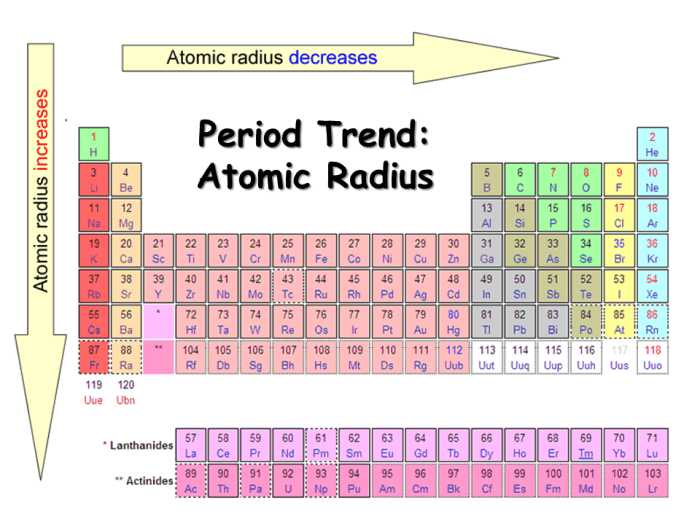
Atomic radius generally decreases from left to right across periods (rows) and increases from top to bottom within groups (columns) in the periodic table. This can be summarized as follows:
- Across periods:As atomic number increases, atomic radius decreases due to the increased nuclear charge.
- Down groups:As you move down a group, the number of energy levels increases, resulting in a larger distance between the nucleus and the outermost electrons, leading to an increase in atomic radius.
Exceptions to Periodic Trends, Periodic trends worksheet atomic radius
There are a few exceptions to the general periodic trends in atomic radius, including:
- Lanthanide contraction:The atomic radii of lanthanides (elements 57-71) are smaller than expected due to poor shielding of the 4f electrons.
- Transition metals:Transition metals (groups 3-12) have relatively small atomic radii compared to their neighboring elements due to the increased effective nuclear charge caused by the incomplete d orbitals.
Applications of Atomic Radius Trends
Knowledge of atomic radius trends has various applications in chemistry, such as:
- Predicting chemical properties:Atomic radius influences properties like ionization energy, electronegativity, and bond length.
- Understanding reactivity:Smaller atomic radii result in stronger interatomic interactions, leading to higher reactivity.
- Designing materials:The atomic radii of elements can guide the selection of materials with specific properties for applications such as catalysis, electronics, and energy storage.
FAQ Resource
What is atomic radius?
Atomic radius is a measure of the size of an atom, specifically the distance from the nucleus to the outermost electron shell.
How does atomic number affect atomic radius?
As atomic number increases across a period, the number of protons in the nucleus increases, leading to a stronger attraction for the electrons. This results in a decrease in atomic radius.
What is the shielding effect?
The shielding effect refers to the ability of inner electrons to reduce the attraction between the nucleus and the outermost electrons. This effect weakens as you move down a group, resulting in an increase in atomic radius.